Bonanza Offer FLAT 20% off & $20 sign up bonus Order Now
Discuss about the Information System Project Management.
Medical Affair Department comprise of the medical experts having an unparalleled experience about projects. Their diligence in medical affairs helps in maintaining clinical performances (PSI, 2016). Such groups are forming essential part of the today’s bio pharmaceutical industry as they will be providing their assistance through availing the services of regulatory agencies, hospital consultants, key opinion leaders (KOL’s) having a great scientific and medical knowledge about the values and the correct usages of products. The professionals working in Medical Affair Department were striving for the highest scientific integrity, so that they will be able to produce the successful clinical trials and provide their valuable contribution in the growth of market. Their activities were overlapping with the sales, clinical growth, Medical marketing and customer services. Now the team members of the Department has prepared a Medical Monitoring Plan also known as Safety Management Plan that will provide detailed instructions and explicit information for the safety reporting progress and study specific medical reporting process (Inventiv Health Vlinical, 2016).
The technical feasibility of the Medical Affair Department can be outlined through its activities where project management group of the department were providing their services for standing out from other CROs of the industry. The staff members were hardly wired for meeting the study timelines and driving the goals of the movement over forwarded and focused directions. They were covering the areas where quality of enrolment and hard deliverables is everything (Crowley-Nowick, 2013). The project managers of the industry are known for their quality of service as on-time project delivery is the compulsory part of their working. Their 95% of success is drawn by the Patient Enrolment Expertise that has been completed on timely basis and ahead of the schedule, year after year. This program took place in three phases in which they have achieved their goals for multiple indications and trial complexities consistently from Phase I to Phase III (Kinapse , 2011).
The rising healthcare costs have forced the employers and government to put their reliance on the controllers. The medical industry is facing challenge for the research and development productivity and there is overall risk-averse in regulatory environment (iconplc, 2016). There is heavy pressure on Medical Affair Department for the maintenance of continuous focus over the optimization of medical value and this has made the conditions critical for the survival of pharmaceutical organizations. All the reforms of Medical Affair Department have promised to intensify the trend. The increase in costs and economic value of medical activities took place due to gradual shift in the decision making power of the physicians over the stakeholders, who were ultimately driving the containment of costs. The engagement of key opinion leaders (KLOs) and advisory board have increased the demands of public scrutiny and greater transparency through the financial disclosures. The department is continuously upgrading their understandings of what the value means for the patients and stakeholders in the broader spectrum of society. For example: When patient thinks of out of pocket cost, at the same time physicians keep their focus over the reimbursement (Callan et al., 2014).
One big mistake by the pharmaceutical company led to greater demand for the transparency of data, operations and policies. The increase in level of transparency will exert higher pressure over the pharmaceutical companies levying responsibilities of data dissemination and data generation. Therefore, the Medical affair Department has channelized some methods in which the companies need to prepare more detailed patient data that will form the basis for the trials of approved drugs and will discontinue the investigation that are easily accessible by the researchers (Diaglobal, 2016). Data management is the core in the clinical trial services. Its implementation in the day to day operational activities standardizes and processes consistent guarantee and high quality data. The delivery of data is made after getting an assurance of quality. This is ensured through validation and verification of procedures at each stage of the process. Such management of quality drives the acceptance on huge basis. This is not only adopted by Medical Affair Department while it is the global approach that is recognized through global compliance (GI LEAD, 2016).
Given below are some of the specifications providing review about the capabilities of Medical Affair Department:
Presently, the medical affair department is handling the enquiries manually but it was found that through Medical Enquiries Database assistance can be provided to the staff in daily work and allow them to respond towards the medical enquiries efficiently and effectively. The scope of the project will be described in the light of proposed system and it is as follows:
The objective of this project is to:
The functional specifications of the project are determined through defining about the instances and main theme of the study. The important function of Medical Affairs Department is to answer the medical enquiries about the medical products. Such information is usually received from the internal customers such as representatives as well as external customers that involves list of persons like retail and hospital pharmacists, doctors, consumers, drug information centres and others. The medical enquiries are received via fax, telephone and letter or in person. It ranges from very simple questions to the complex questions that can be easily resolved through answering over the telephone or either takes hours of research for the formulation of responses (pharmiweb, 2013). The responsibilities of the Medical Affair Department have risen with the merger of two pharmaceutical companies. They were now needed to handle the large number of enquiries with the increased portfolio of products. Presently, for resolving the enquiries, they record the receipt and reply them. The whole system works on paper based environment (Evers et al., 2016).
The non-functional specification will contain brief information about the issues that were addressed in the study. The inefficiencies of paper-based environment not only sidetrack the medical professionals while also creates delay in reimbursements and even requires compromise care. The recording of enquiries and resolving through Medical Enquiries Database would not only streamline the workflow while also reduces the operating cost (Hostetler, 2016). Apart from it, it will also enhance the experience of the patients. This strategy will not only transform the medical practices while also helps in making effective management of records. But such practices often require large investment and compelling for motivation through implementation of large scale solution. With the use of new technological innovations not only make the system efficient while also help in spending more time with the patients. In the paperless environment, many general practices can be developed such full integration of Window-based system including an access towards the full electronic medical records (Brooks, 2016).
The modification in the traditional working environment creates many complexities. Similarly, here is the same case. The exploration of Medical Enquiries Database System, however reduce dependence on paper based environment but it will require some ambulatory care settings and requires proposal of some reasonable steps. One of the most critical steps in this process is to choose the knowledgeable and an experienced partner who will analyse the whole documentation system and helps in proposing the plan that will be most suitable in such kind of practices. This will help in gaining an insight that which benefit will be more suitable in particular medical practices and keep the favour on the part of patients. Another emerging issue is related with the resistance of staff for the change. The clinical environment is already highly interrupted and non-linear environment. Physicians may deny for making the changes because after the exposure of medical facilities through paperless environment, they may require to spend substantial amount of time over the computers and less over the medical care. Any solution that consumes higher time period is apt for the resistance.
The project has discussed about a Medical case study of the Medical Affair Department that is compelling about the common issues of paper based system for the recording of enquiries. The topic has been evaluated in the light of feasibility study containing technical feasibility, economic feasibility and operational feasibility.
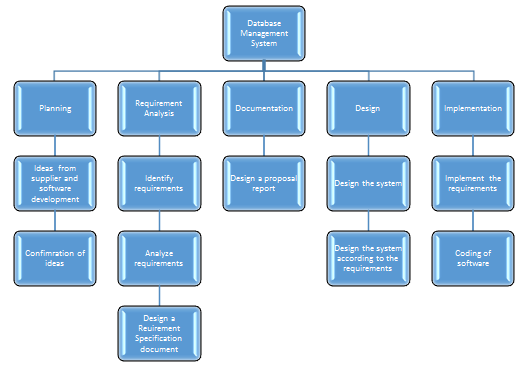
Figure 1: Work Breakdown Structure
Further analysis is done through defining some points under requirement specification containing information about scope of the project, objectives of the project, functional and non-functional specifications and constraints of the project. This study is quite useful for the further researches because it is demonstrating about the challenges of the paper based environment and the issues in maintenance of records of enquiries. It is further suggested about some of the strategies through which an attention towards the maintenance of the health records system can be gathered.
References
Brooks, C., 2016. Document Management Systems: A Buyer's Guide. [Online] Available at: http://www.businessnewsdaily.com/8026-choosing-a-document-management-system.html [Accessed 24 August 2016].
Callan, R.S. et al., 2014. Effectiveness and feasibility of utilizing E4D technology as a teaching tool in a preclinical dental education environment. Journal of dental education, 78(10), pp.1416-23.
Crowley-Nowick, P.C.-N.J.S.a.P., 2013. The Role of Medical Affairs in Moving from R&D to Commercialization. [Online] Available at: http://www.bioprocessintl.com/analytical/qa-qc/the-role-of-medical-affairs-in-moving-from-randd-to-commercialization-341871/ [Accessed 24 September 2016].
Diaglobal, 2016. Building a competent Medical Affairs team to support business needs: A personal perspective. Diaglobal.
Evers, M. et al., 2016. What does a Medical Affairs Manager Do? McKinsey & Company.
GI LEAD, 2016. Senior Manager, Medical Affairs, Health Economics and Outcomes Research. [Online] Available at: https://gilead.avature.net/careers/FolderDetail/Foster-City-California-United-States-Associate-Director-Medical-Affairs-Health-Economics-and-Outcomes-Research/31582 [Accessed 24 September 2016].
Hostetler, R., 2016. A Realistic Transition Toward the “Paperless” Medical Practice. Healthcare Lanier Worldwide, Inc.
iconplc, 2016. Medical Affairs. [Online] Available at: http://www.iconplc.com/services/clinical-research-services/medical-safety-services/medical-affairs/ [Accessed 24 September 2016].
Inventiv Health Vlinical, 2016. Medical & Scientific Affairs. [Online] Available at: http://www.inventivhealthclinical.com/phase-ii-iii-clinical-trials-medical-and-scientific-affairs.htm [Accessed 24 September 2016].
Kinapse , 2011. Performance Management in Medical Affairs. Kinapse Consulting.
pharmiweb, 2013. What does a Medical Affairs Manager Do? [Online] Available at: http://www.pharmiweb.com/features/feature.asp?ROW_ID=1661#.V-ZXnih97IW [Accessed 24 September 2016].
PSI, 2016. Medical Affairs Division. [Online] Available at: http://www.psi-cro.com/full-service-cro/medical-affairs/ [Accessed 24 September 2016].
Upload your Assignment and improve Your Grade
Boost Grades